Von Karstedt Lab
Cell Death and Cancer Evolution
The evolution of cancer is tightly regulated by repeated cycles of cell proliferation, selection of the fittest clone and cell death of all weaker clones. Thereby, cancers strictly follow the rule of “Darwinian” selection throughout their existence. Although the induction of cell death in a cancer cell is wanted from a therapeutic viewpoint, it is also the driver behind selection for the most aggressive cancer cell clone. Therefore, it is our mission to provide insights into cell death mechanisms as a driver of selection during early cancer evolution in order to turn selection into efficient eradication of tumours.
Our research focus:
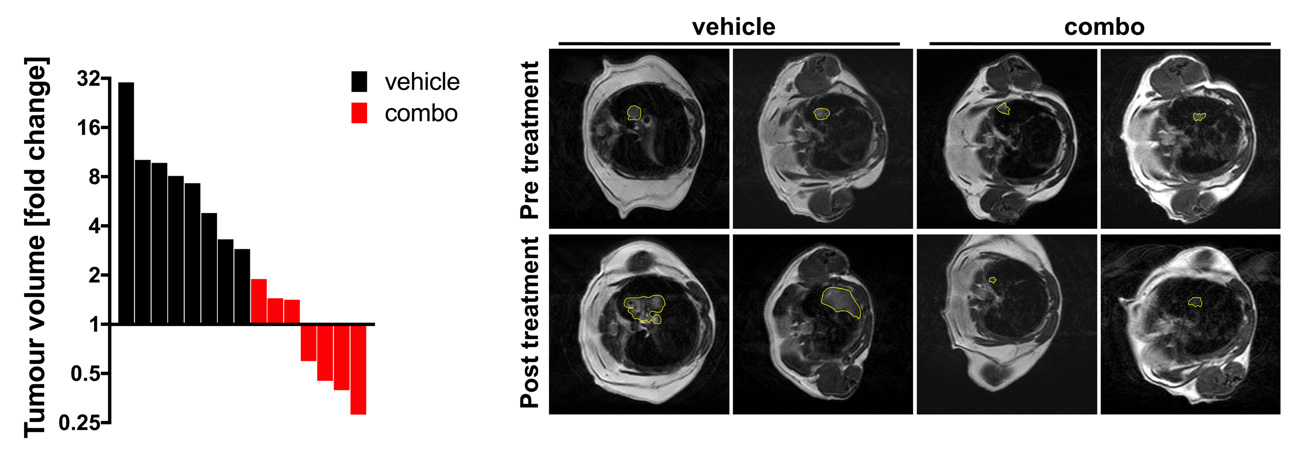
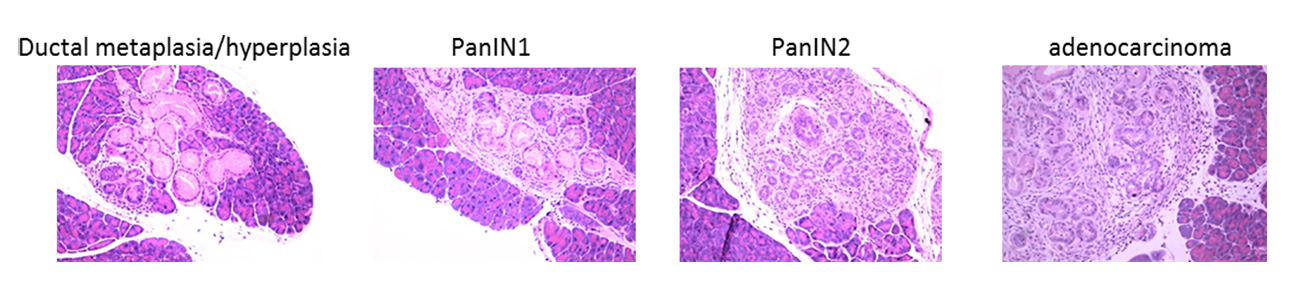
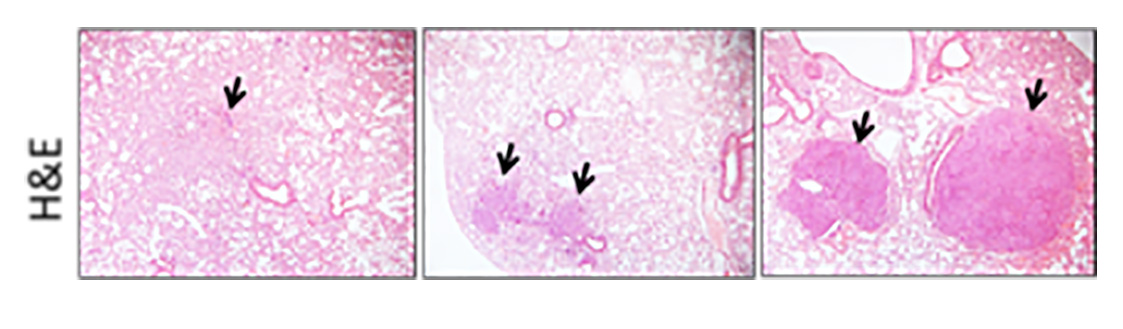
In order to maintain healthy tissue homeostasis, normal cells must proliferate if needed but also die when asked. If this fine balance is perturbed, tissues can accumulate renegade cells which have the potential to transform and become cancer. Malignant cancer cells within a tumour avoid healthy tissue regulation resulting in constantly proliferating cancer cells with acquired cell death resistance. To achieve these cancerous hallmark features, early premalignant lesions likely undergo many cycles of fractional killing of premalignant cells which inevitably will select for the most resistant subclones. Thereby, unintentionally anti-cancer immune cells might contribute to the selection of the most aggressive cellular clone by creating constant selective pressure through cell death induction. To kill tumour cells, immune effector cells can make use of tumor necrosis factor (TNF) superfamily ligands such as TNF, CD95L and TNF-related apoptosis-inducing ligand (TRAIL). Binding of these ligands to their cognate receptors expressed on tumour cells can trigger regulated types of cell death. In addition to direct ligand/receptor interaction-triggered regulated cell death such as extrinsic apoptosis and necroptosis, cancer cells can also undergo selective pressure from oxidative damage-associated regulated cells death such as ferroptosis, evasion of which is selected for in lung cancers. To identify actionable vulnerabilities in these selection processes, the von Karstedt lab investigates the function of different modes of cell death in a) the selection of cancers driven by oncogenic KRAS expression and b) selection of never-KRAS mutated small cell lung cancer (SCLC).
Our goals:
By unravelling mechanisms of selection-of-the-fittest in lung and pancreatic cancer, we anticipate to identify cell death pathways which remain amenable to therapeutic triggering. These insights will be vital for the development of novel therapeutic strategies targeting these mechanisms.
Our approach:
The von Karstedt lab combines the use of genetically engineered and cigarette smoke-associated carcinogen-induced mouse models to explore the role of cell death-mediated selection during early tumour development. These approaches are underpinned by cell biological, multi-omics and biochemical studies on mechanistic aspects in cell culture.
Principal Investigator
Team
Most important publications
- Anna Willms, Hella Schupp, Michelle Poelker, Torsten Hartwig, Tim Krichel, Jürgen Fritsch, Steven Singh, Henning Walczak, Silvia von Karstedt, Heiner Schäfer, Anna Trauzold. „TRAIL-R2 – a novel negative regulator of p53“. Cell Death and Disease, (accepted in principle).
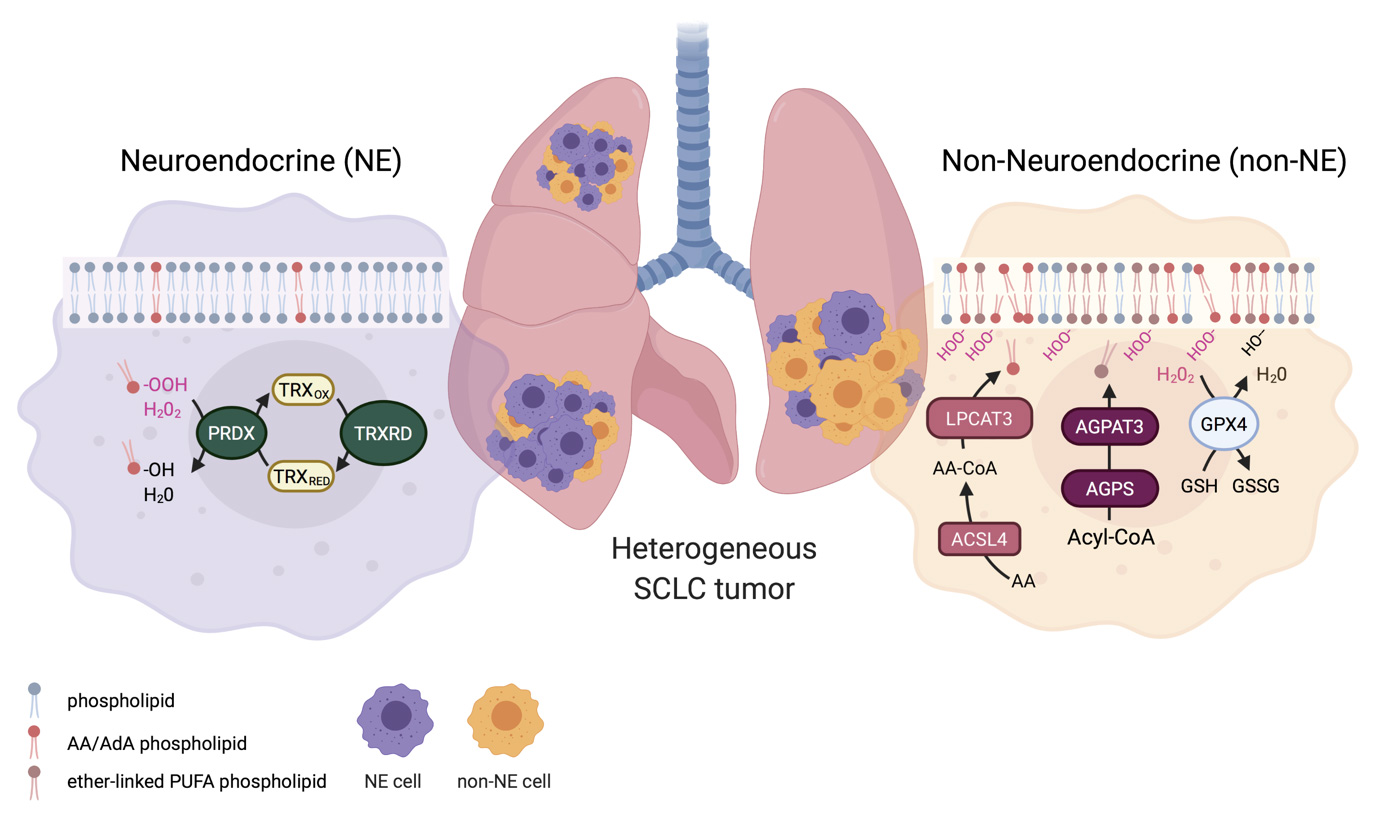
- Christina M. Bebber, Emily S. Thomas, Zhiyi Chen, Jenny Stroh, Ariadne Androulidaki, Anna Schmitt, Michaela N. Höhne, Lukas Stüker, Cleidson de Pádua Alves, Armin Khonsari, Marcel A. Dammert, Fatma Parmaksiz, Hannah L. Tumbrink, Filippo Beleggia, Martin L. Sos, Jan Riemer, Julie George, Susanne Brodesser, Roman K. Thomas, H. Christian Reinhardt and Silvia von Karstedt. Ferroptosis response segregates small cell lung cancer (SCLC)
neuroendocrine subtypes. Nature Communications 12, 2048 (2021).
- Lohans Pedrera, Rafael A. Espiritu, Uris Ros, Josephine Weber, Anja Schmitt, Jenny Stroh, Stephan Hailfinger, Silvia von Karstedt and Ana J. Garcia-Saez. Ferroptotic pores induce Ca2+ fluxes and ESCRT-III activation to modulate cell death kinetics. Cell Death and DifferentiationNature Publishing Group; 2020 Dec 17;149:1–14.
- Clemente LP, Rabenau M, Tang S, Stanka J, Cors E, Stroh J, Culmsee C, Karstedt von S. Dynasore Blocks Ferroptosis through Combined Modulation of Iron Uptake and Inhibition of Mitochondrial Respiration. Cells 2020, Vol. 9, Page 2259. Multidisciplinary Digital Publishing Institute; 2020 Oct 1;9(10):2259.
- Lim, J.K.M., Delaidelli, A., Minaker, S.W., Zhang, H.-F., Colovic, M., Yang, H., Negri, G.L., Karstedt, von, S., Lockwood, W.W., Schaffer, P., Leprivier, G., Sorensen, P. H. (2019). Cystine/glutamate antiporter xCT (SLC7A11) facilitates oncogenic RAS transformation by preserving intracellular redox balance. PNAS. 1092, 201821323.
- Mert, U., Adawy, A., Scharff, E., Teichmann, P., Willms, A., Haselmann, V., Colmorgen, C., Lemke, J., Karstedt, von, S., Fritsch, J. & Trauzold, A. TRAIL Induces Nuclear Translocation and Chromatin Localization of TRAIL Death Receptors. Cancers (Basel)11, 1167 (2019).1.
- Heilmann, T., Vondung, F., Borzikowsky, C., Szymczak, S., Krüger, S., Alkatout, I., Wenners, A., Bauer, M., Klapper, W., Röcken, C., Maass, N., Karstedt, von, S., Schem, C. & Trauzold, A. Heterogeneous intracellular TRAIL-receptor distribution predicts poor outcome in breast cancer patients. J Mol Med97, 1155–1167 (2019).
- Krishna K. Kolluri, Constantine Alifrangis, Neelam Kumar, Stacey Price, Magali Michaut, Steven Williams, Syd Barthorpe, Yuki Ishii, Howard Lightfoot, Sara Busacca, Annabel Sharkey, Zhenqiang Yuan, Elizabeth K. Sage, Sabarinath Vallath, John Le Quesne, David A. Tice, Doraid Alrifai, Silvia von Karstedt, Antonella Montinaro, Naomi Guppy, David A. Waller, Apostolos Nakas, Robert Good, Alan Holmes, Henning Walczak, Dean A. Fennell, Mathew J. Garnett, Francesco Iorio, Lodewyk F. A. Wessels, Ultan McDermott, Sam M. Janes; Loss of functional BAP1 augments sensitivity to TRAIL in cancer cells. Elife. 2018 Jan 18;7. pii: e30224. doi: 10.7554/eLife.30224.
- Hartwig, T.*, Montinaro, A.*, von Karstedt, S.*, Sevko, A., Surinova, S., Chakravarthy, A., Taraborrelli, L., Draber, P., Lafont, E., Arce Vargas, F., Bahrawy, M. A., Quezada, S. A. and Walczak, H; The TRAIL-induced cancer secretome promotes a tumor-supportive immune-microenvironment via CCR2. Molecular Cell65, 730–742.e5 (2017). * equal contribution.
- Zinngrebe, J.*, Rieser, E.*, Taraborrelli, L., Peltzer, N., Ren, H., Kovács, I., Endres, C., Draber, P., Darding, M., von Karstedt, S., Lemke, J., Dome, B., Bergmann, M., Ferguson, B. J., Walczak, H.; LUBAC deficiency perturbs TLR3 signaling to cause immunodeficiency and autoinflammation. Journal of Experimental Medicine 213, 2671-2689, doi:10.1084/jem.20160041 (2016). * equal contribution.
- von Karstedt, S., Conti, A., Nobis, M., Montinaro, A., Hartwig, T., Lemke, J., Legler, K., Annewanter, F., Campbell, A.D., Taraborrelli, L., Grosse-Wilde, A., Coy, J. F., Bahrawy, M. A., Bergmann, F., Koschny, R., Werner, J., Ganten, T. M., Schweiger, T., Hoetzenecker, K., Kennessey, I., Hegedüs, B., Bermann, M., Hauser, C., Egberts, J.-H., Becker, T., Röcken, C., Kalthoff, H., Trauzold, A., Anderson, K. I., Sansom, O. J. and Walczak, H.; Cancer Cell-Autonomous TRAIL-R Signaling Promotes KRAS-Driven Cancer Progression, Invasion, and Metastasis. Cancer Cell 2015; 27:561-573.
- Tuthill MH, Montinaro A, Zinngrebe J, Prieske K, Draber P, Prieske S, T Newsom-Davis, S von Karstedt, J Graves, H Walczak;TRAIL-R2-specific antibodies and recombinant TRAIL can synergise to kill cancer cells. Oncogene2015; 34(16): 2138-44.
- Lemke J, von Karstedt S, Abd El Hay M, Conti A, Arce F, Montinaro A, K Papenfuss, MA El-Bahrawy and H Walczak; Selective CDK9 inhibition overcomes TRAIL resistance by concomitant suppression of cFlip and Mcl-1. Cell Death and Differentiation 2014; 21(3): 491-502.
Patents
Named inventors: H.Walczak, S. von Karstedt; Methods for treating cancer (publication date 08.01.2015; WO2015001345)