Peltzer Lab
Cell Death and Inflammation in Disease
Cell death is important for physiological processes, such as embryonic development, immune tolerance, fight against pathogens, etc. However, uncontrolled cell death can lead to chronic inflammation, tissue damage and autoimmunity. Cell death-induced chronic inflammation is also associated with cancer. Different cell death programmes release specific factors that impact on immune responses and tumorigenesis in a distinctive manner. Our goal is to study the impact of different forms of programmed cell death on disease pathogenesis and to exploit this knowledge for therapeutic purposes by controlling the levels of cell death and by rewiring cell death programs to the host’ advantage.
Our research focus:
Organisms can sense both damage and pathogens via the so-called damage- and pathogen-associated molecular patterns (DAMPs and PAMPs, respectively) that are recognised by Pattern-recognition receptors (PRRs). Triggering of PRRs results in the induction of inflammatory cytokines and chemokines including, among others, TNF, IL-1b and type I IFNs. These cytokines play crucial roles in triggering innate immune responses by binding to their respective receptors, including TNF receptor (TNFR) superfamily (SF) members.
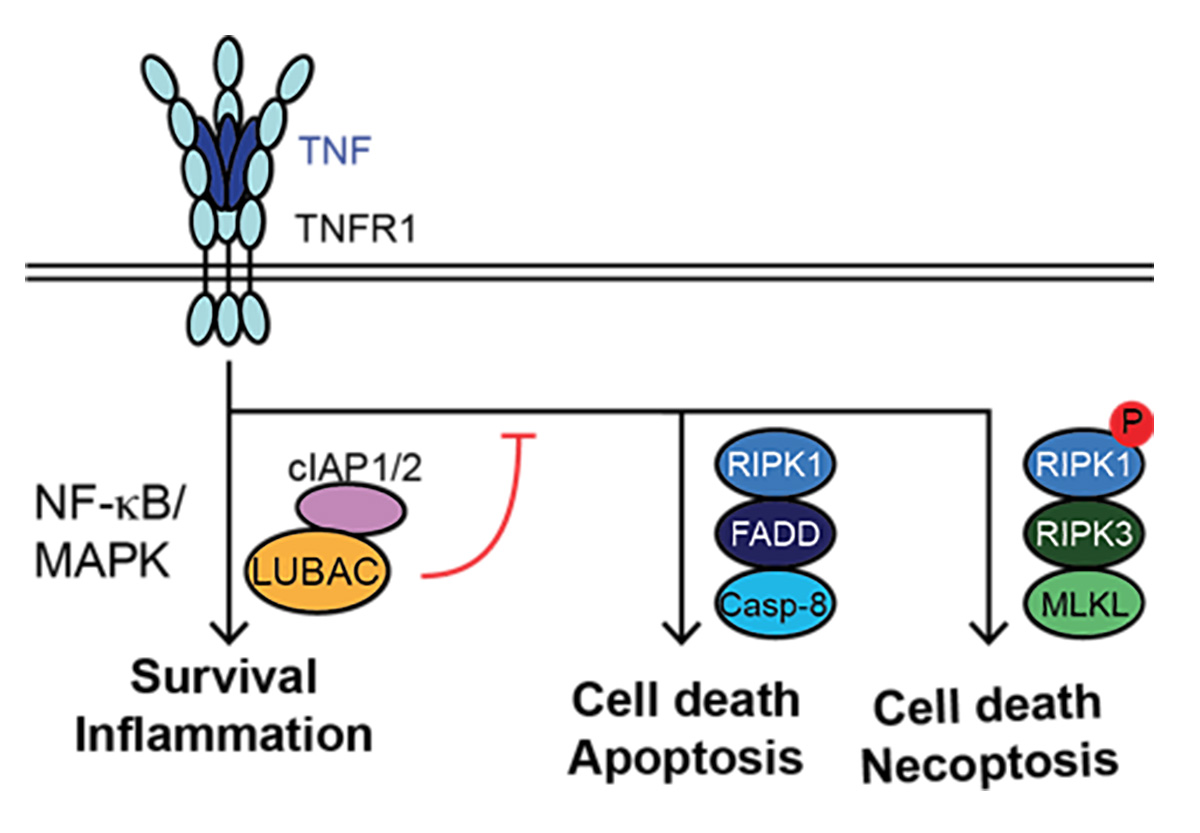
Binding of TNF to its cognate receptor TNFR1 results in downstream cascades that induce: i) inflammation/survival via LUBAC and cIAP1/2, by activating NF-kB/MAPK-mediated gene expression, ii) apoptosis via FADD/RIPK1/caspase-8 or, iii) necroptosis via RIPK1 autophosphorylation, RIPK3 and MLKL (Fig. 1). In normal physiology, TNFR1-signalling output is skewed towards inflammation/survival; however, in autoimmune disorders or pathological conditions this balance is shifted towards cell death induction.
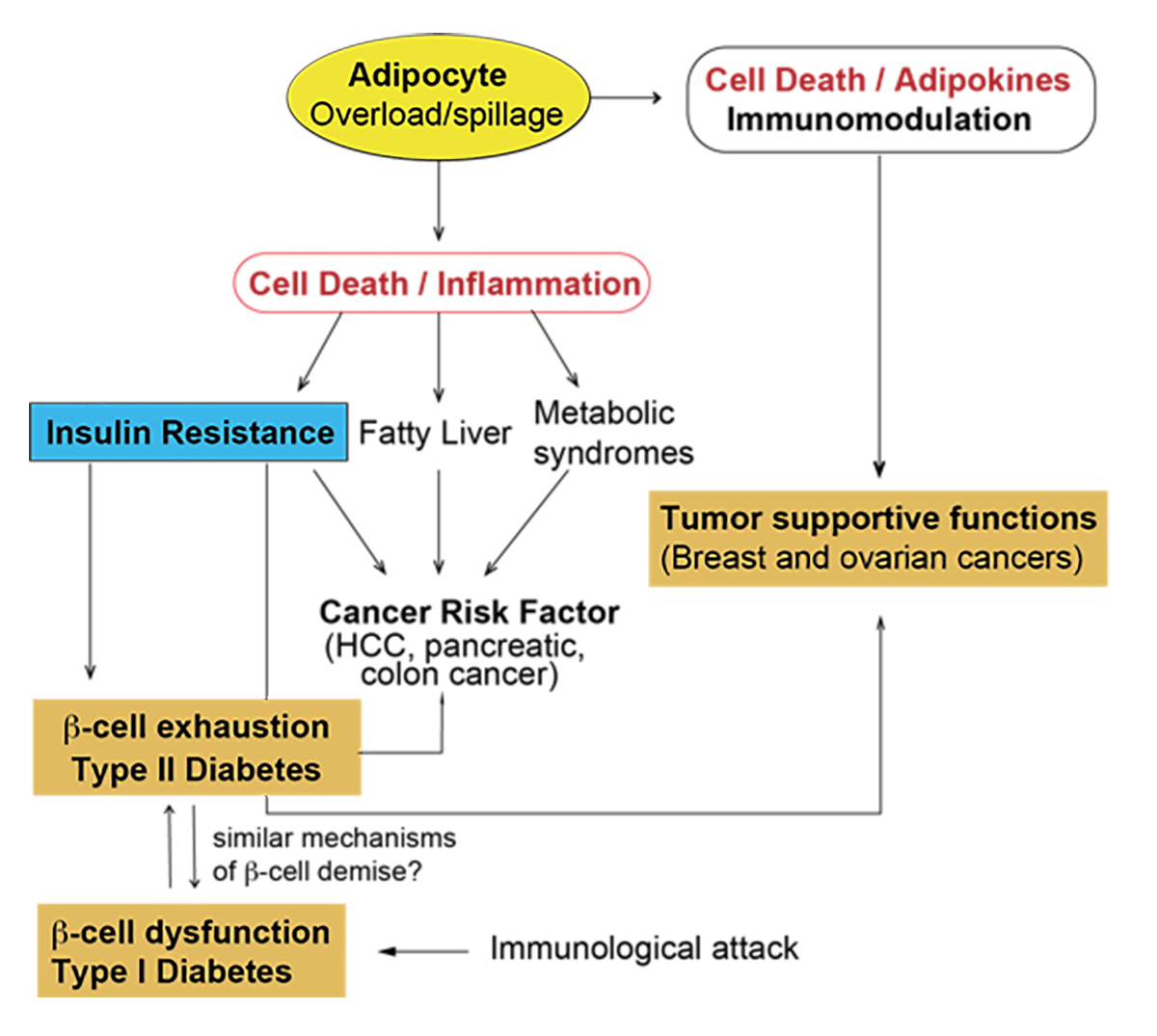
Our group studies how different modes of inflammatory cell death induced by immune receptors impact on autoimmunity, chronic inflammation and tumorigenesis. One aspect of our research plan focuses on the role of cell death in inducing or perpetuating autoimmunity in a model of Type I Diabetes. Another aspect is the study of cell death in obesity-induced inflammation and its associated metabolic complications such as insulin resistance and Type 2 Diabetes (T2D). We are also interested in understanding how tumor cells regulate cell death programs to their own advantage and design strategies to to characterize new tumor suppressors but also to discover novel tumor vulnerabilities.
Our goals:
We aim to study the mechanisms dictating β-cell death in the context of autoimmunity and during obesity and find common and unique features in T1/2D. Furthermore, we aim to explore the interplay between cell death and obesity-induced inflammation and understand the contribution of adipocyte death during obesity in metabolic syndromes (including T2D) and in the tumor microenvironment (Fig. 2). Our mission is to understand disease aetiology at the molecular level to identify potential treatments for autoimmune and inflammation-driven disorders, including cancer.
Our approach:
Our laboratory has a number of genetically engineered mouse models to underpin the role of cell death in different pathophysiological conditions. This is combined with a wide range of imaging, molecular and cell biology approaches for mechanistic characterisation of the role of different programmed cell death programmes in health and disease.
Principal Investigator
Most important publications
- Peltzer, N.*, and Walczak, H*. Cell Death and Inflammation - A Vital but Dangerous Liaison. Trends in immunology(IF: 13) 40, 387-402, 2019
- Peltzer, N*., Darding, M*., Montinaro, A., Draber, P., Draberova, H., Kupka, S., Rieser, E., Fisher, A., Hutchinson, C., Taraborrelli, L., Hartwig, t., Lafont, E., Haas, T.L., Shimizu, Y., Böiers, C., Sarr, A., Rickard, J., Alvarez-Diaz, S., Ashworth, M.T., Beal, A., Enver, T., Bertin, J., Kaiser, W., Strasser, A., Silke, J., Bouillet, P., Walczak, H. LUBAC is essential for embryogenesis by preventing cell death and enabling haematopoiesis. Nature557: 112–117, 2018.
- Taraborrelli, L*., Peltzer, N*., Montinaro, A., Kupka, S., Rieser, E., Hartwig, T., Sarr, A., Darding, D., Draber, P., Haas, T.L., Akarca, A., Marafioti, T., Pasparakis, M., Bertin, J., Gough, P.J., Bouillet, P., Strasser, A., Leverkus, M., Silke, S., Walczak, H. LUBAC prevents lethal dermatitis by combined inhibition of TNF-, TRAIL- and CD95L-mediated cell death. Nature Communications9:3910, 2018
- Shimizu, Y., Peltzer, N., Sevko, A., Lafont, E., Sarr, A., Draberova, H., Walczak, H. The linear ubiquitin chain assembly complex acts as a liver tumour suppressor and inhibits hepatocyte apoptosis and hepatitis. Hepatology65(6): 1963-1978, 2017.
- Peltzer N, Darding M, Walczak H. Holding RIPK1 on the Ubiquitin Leash in TNFR1 Signaling. Trends Cell Biol(IF: 13.5) 26(6):445-61, 2016
- Peltzer, N*., Rieser, E.*, Taraborrelli, L., Draber, P., Darding, M., Pernaute, B., Shimizu, Y., Daboh, A., Draberova, H., Montinaro, A., Martinez-Barbera, J.P., Silke, J., Rodriguez, T.A. and Walczak, H. HOIP deficiency caused embryonic lethality by aberrant TNFR1- mediated endothelial cell death. Cell Reports 9: 153-165, 2014
- Liccardi, G., Ramos Garcia, L., Tenev, T., Annibaldi, A., Legrand, AJ., Robertson, D, Feltham, F., Anderton, H., Darding, M., Peltzer, N, Dannappel, M., Schünke, H.,Fava, L., Haschka, M.D., Glatter, T, Nesvizhskii, T., Schmidt, A., Harris, P.A., Bertin, J., Gough, P., Villunger, A., Silke, J., Pasparakis, M., Bianchi, K. and Meier, P. RIPK1 and Caspase-8 Ensure Chromosome Stability Independently of Their Role in Cell Death and Inflammation. Molecular Cell73, 11.010, 2019.
- Giampazolias, E., Zunino, B., Dhayade, S., Bock, F., Cloix, C., Cao, K., Roca, A., Lopez, J., Ichim, G., Proïcs, E., Rubio-Patiño, C., Fort, L., Yatim, N., Woodham, E., Orozco, S., Taraborrelli, L., Peltzer, N., Lecis, D., Machesky, L., Walczak, H., Albert, ML., Milling, S., Oberst, A., Ricci, JE., Ryan, KM., Blyth, K., Tait, SWG. Mitochondrial permeabilization engages NF-κB-dependent anti-tumour activity under caspase deficiency. Nat Cell Biol19(9): 1116-1129, 2017.
- Zinngrebe, J., Rieser, E., Taraborrelli, L., Peltzer, N., Hartwig, T., Ren, H., Kovács, I., Endres, C., Draber, P., Darding, M., Karstedt, S., Lemke, J., Dome, B., Bergmann, M., Ferguson, B., Walczak, H. LUBAC deficiency perturbs TLR3 signaling to cause immunodeficiency and autoinflammation. The Journal of Experimental Medicine213(12):2671-2689, 2016
- Khalil, H.*, Peltzer, N*., Walicki, J., Yang, JY., Dubuis, G., Gardiol, N., Held, W., Bigliardi, P., Marsland, B., Liaudet, L. and Widmann, C. Caspase-3 protects stressed organs against cell death. Molecular and Cellular Biology32(22):4523-33, 2012
*co-first authors